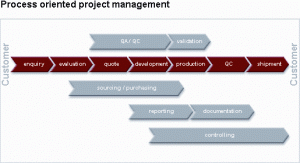
herapak’s Clinical Trial Services are governed by a Master Services Agreement (MSA) and subsequent Work Orders for each protocol. Once the Confidentiality Disclosure Agreement (CDA) is in place, and concurrent with the review of the MSA, the protocol or project supply requirements are reviewed by Business Development so that a Scope of Work proposal (Work Order) can be prepared. The Work Order lists all the clinical supplies and services that will be provided for each protocol along with a Bill of Materials breakdown for each unique kit type. Typical components accounted for on a Work Order will include the necessary specimen collection kits, ancillary bulk supplies, sample shipping supplies, chemical reagents, and any print material such as tube labels, requisition forms, lab manuals, airway bills and any other custom documents.
All customers are assigned a dedicated Therapak Project Manager for each protocol once the initial scope of services is agreed on by both parties. Our skilled Project Managers will manage all remaining aspects of setting up the study and work closely with client’s clinical team to process the study according to the agreed upon requirements and protocol launch timelines. Among other things, Therapak’s Project Manager will create all of the necessary production documentation for each kit style, prepare tube label templates for customer’s review and approval, and set-up the protocol in Therapak’s proprietary online ordering system called CT-Online for managing and tracking all aspects of site supply orders.
Please contact info@site_2050c169-3162-49d2-8164-08904a9c57ee to discuss the clinical supply needs for your upcoming study.